The RESCUEicp Study: Decompressive craniectomy for traumatic intracranial hypertension
Severe traumatic brain injury (TBI) is a significant medical condition, often with poor clinical outcomes. Raised intracranial pressure (ICP) following a severe TBI is common, from both generalised cerebral oedema and surgically amenable pathology such as haemorrhagic mass lesions or hydrocephalus. Raised ICP can result in deleterious secondary brain injury through its impact in cerebral perfusion pressure, and so its measurement and treatment remains a common part of the management of severe TBI. A critical care challenge is how best to manage these patients with refractory high ICP, and decompression of the cranial cavity via a craniectomy is commonly used as one of the last resorts. The decompressive craniectomy (DECRA) trial had suggested unfavourable outcomes with primary decompression (i.e. anticipatory) and this study aimed to assess this current role as a last-ditch rescue therapy.
What did they do?
Population: Hospitals with acute neuroscience care. 52 centres in 20 countries, but 71% from the UK.
Inclusion criteria: Age 18 – 65, TBI with abnormal CT scan, ICP monitoring in place, refractory high ICP (ICP > 25mmHg for 1-12 hours despite stage 1 and 2 measures e.g. head up, sedation, cooling, osmotherapy).
Exclusion: Fixed dilated pupils, bleeding diathesis, injury deemed to be unsurvivable.
Intervention: Decompressive craniectomy – exact type at the discretion of the operating surgeon. Recommended within 4-6 hours of randomisation.
Control: Maximal medical therapy. Generally, barbiturate coma. Stage 1 and 2 therapies continued in both groups. Crossover allowed in both groups.
Primary outcome: Extended Glasgow Outcome Scale (GOS-E) at 6 months (8 point scale from death to no diability). Dichotomy of good vs bad outcome – Good outcome being GOS-E of upper severe disability or better. Link: https://www.thomsonrogers.com/wp-content/uploads/2015/10/Glasgow-Outcome-Scale-Extended.pdf
Secondary outcomes: GOS-E at 12 and 24 months. Death at 6,12, and 24 months. Quality of life at 6,12, and 24 months. Glasgow Coma Score (GCS) at discharge. Assessment of ICP control. Time on ICU. Time to hospital discharge.
Stats: Telephone randomisation in 1:1 ratio, permuted blocks of random sizes. Modified intention to treat analysis. Power calculation. Difference between the groups assessed by an ordinal analysis method.
Inclusion criteria: Age 18 – 65, TBI with abnormal CT scan, ICP monitoring in place, refractory high ICP (ICP > 25mmHg for 1-12 hours despite stage 1 and 2 measures e.g. head up, sedation, cooling, osmotherapy).
Exclusion: Fixed dilated pupils, bleeding diathesis, injury deemed to be unsurvivable.
Intervention: Decompressive craniectomy – exact type at the discretion of the operating surgeon. Recommended within 4-6 hours of randomisation.
Control: Maximal medical therapy. Generally, barbiturate coma. Stage 1 and 2 therapies continued in both groups. Crossover allowed in both groups.
Primary outcome: Extended Glasgow Outcome Scale (GOS-E) at 6 months (8 point scale from death to no diability). Dichotomy of good vs bad outcome – Good outcome being GOS-E of upper severe disability or better. Link: https://www.thomsonrogers.com/wp-content/uploads/2015/10/Glasgow-Outcome-Scale-Extended.pdf
Secondary outcomes: GOS-E at 12 and 24 months. Death at 6,12, and 24 months. Quality of life at 6,12, and 24 months. Glasgow Coma Score (GCS) at discharge. Assessment of ICP control. Time on ICU. Time to hospital discharge.
Stats: Telephone randomisation in 1:1 ratio, permuted blocks of random sizes. Modified intention to treat analysis. Power calculation. Difference between the groups assessed by an ordinal analysis method.
What did they find?
Numbers: 2008 patients assessed, with 408 successfully randomised. 10 patients withdrawn due to consent issues. 9 lost to follow up before primary outcome. 201 patient in surgical arm, 188 in medical arm. No significant difference between groups other than fewer patients with drug/alcohol abuse in the medical arm. Patients primarily male (81%), with a mean age of 33.5 years.
Intervention: 92.6% of the surgical arm underwent decompressive craniectomy. Median time from randomisation to treatment was 2.2 hours. 87.2% of the medical arm received a barbiturate infusion. Median time to treatment was 1.5 hours with a median duration of 53 hours. 37.2% of the medical group crossed over and also underwent a decompressive craniectomy.
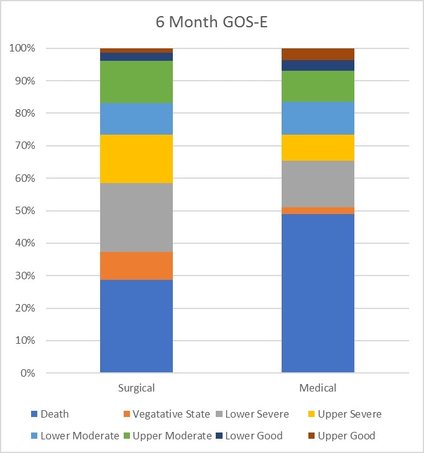
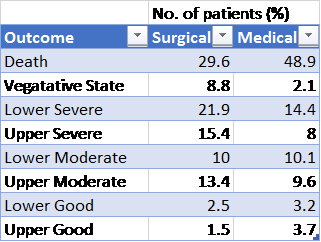
Results – Primary outcome: A significant difference in GOS-E between the two arms (p=0.005). However, goodness of fit test not passed so results presented descriptively. The results are outline in the table below. Favourable outcomes occurred in 42.8% of the surgical group and 34.6% of the medical group (p=0.12)
Secondary outcome: At 12 months, the relationship between the groups was similar to that at 6 months. A favourable outcome occurred in 45.4% of the surgical group and 32.4% of the medical group. Control of ICP was better in the surgical group based on the 5 parameters used. Adverse events occurred more frequently in the surgical group compared to the medical group - 16.3% vs 9.2 % (p=0.03). No difference in the time to discharge from the ICU between groups.
Intervention: 92.6% of the surgical arm underwent decompressive craniectomy. Median time from randomisation to treatment was 2.2 hours. 87.2% of the medical arm received a barbiturate infusion. Median time to treatment was 1.5 hours with a median duration of 53 hours. 37.2% of the medical group crossed over and also underwent a decompressive craniectomy.
Results – Primary outcome: A significant difference in GOS-E between the two arms (p=0.005). However, goodness of fit test not passed so results presented descriptively. The results are outline in the table below. Favourable outcomes occurred in 42.8% of the surgical group and 34.6% of the medical group (p=0.12)
Secondary outcome: At 12 months, the relationship between the groups was similar to that at 6 months. A favourable outcome occurred in 45.4% of the surgical group and 32.4% of the medical group. Control of ICP was better in the surgical group based on the 5 parameters used. Adverse events occurred more frequently in the surgical group compared to the medical group - 16.3% vs 9.2 % (p=0.03). No difference in the time to discharge from the ICU between groups.
Graph and table describing GOS-E at 6 months
Is it any good?
Overall: Yes, though there are some problems with implementing the results into practice.
Strengths: Decent size. Pragmatic approach to study design. Use of GOS-E appears to be a valid and useful outcome measure. Blinding of assessors of outcome to the intervention. Time to intervention was suitably rapid in both groups. Early consent for study inclusion but allocation not revealed until stage 3 intervention needed.
Weaknesses: The cut-off for favourable vs unfavourable outcome is challenging. The authors in this group have used a different GOS-E level in this trial and it has a significant impact on deciding whether the intervention is beneficial. Crossover between the trial arms had the potential to confuse the results – however, this would likely be to dilute the effect size of the intervention. Clinical teams not blinded to intervention, with potential bias. Possibility of response bias from the way outcome data was collected (post and telephone).
Strengths: Decent size. Pragmatic approach to study design. Use of GOS-E appears to be a valid and useful outcome measure. Blinding of assessors of outcome to the intervention. Time to intervention was suitably rapid in both groups. Early consent for study inclusion but allocation not revealed until stage 3 intervention needed.
Weaknesses: The cut-off for favourable vs unfavourable outcome is challenging. The authors in this group have used a different GOS-E level in this trial and it has a significant impact on deciding whether the intervention is beneficial. Crossover between the trial arms had the potential to confuse the results – however, this would likely be to dilute the effect size of the intervention. Clinical teams not blinded to intervention, with potential bias. Possibility of response bias from the way outcome data was collected (post and telephone).
Final Thoughts
Decompressive craniectomy seems to be effective at reducing ICP and saving lives. However, the result of this appears to be that more patients survive with a ‘worse outcome’, based on the GOS-E, although the determinant of what constitutes a good or bad outcome has some subjectivity. The results are therefore difficult to apply universally, as the significance of this will be patient specific.
Written: Tom Heaton
Reviewed: Not done
6th Feb 2017
Reviewed: Not done
6th Feb 2017
Links & References
- Hutchinson P et al. Trial of decompressive craniectomy for traumatic intracranial hypertension (RESCUEicp Trial). 2016. NEJM. 375: 1119-1130. Avaialble at: http://www.nejm.org/doi/full/10.1056/NEJMoa1605215#t=article